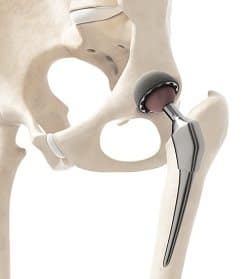
The DePuy Recall and Hip-Replacement Failure Symptoms
In August 2010, DePuy issued a voluntary recall of its ASR TM Total Hip System following research by the UK National Joint Registry showing that revision rates, how often a second surgery was needed, within five years for the device totaled 13 percent or about one revision for every eight patients. This was a significantly higher rate than had previously been reported or acknowledged by the company.
The DePuy device is a metal-on-metal artificial hip, meaning that both the ball and socket are made of metal. The advantage of metal is that it is durable and strong. However, it may release metal ions into the blood stream, which over time can corrode the surrounding bone and soft tissue.
If you have a DePuy hip replacement, the fact that this device is under recall does not necessarily mean that you need to have it replaced. It does mean that you should see your orthopedic surgeon, as he or she may want to monitor your device more closely.
If you do experience symptoms of hip-replacement failure, you may need revision surgery.
Symptoms of Hip-Replacement Failure
Some physical symptoms after a successful hip replacement surgery, such as mild pain and swelling, are not a cause for alarm. Of course, if they worsen over time, you should speak with your surgeon about them. Other symptoms, such as nerve damage or difference in leg length, require immediate treatment but most likely not a device replacement.
The federal Food and Drug Administration, which monitors the safety of regulated medical products, says you should be aware of several symptoms that may be signs your device is failing. These include:
- Pain in the groin and hip area (even after three months following the operation)
- Swelling in the hip area
- Limping or trouble walking
- Noise (clicking, squeaking, etc.) coming from the joint
Occasionally symptoms present in other parts of the body, so also take note of any changes in your general health. If you have a DePuy hip replacement, and you need a revision surgery, it’s time to think about the next step, which includes legal action to recover for your damages.
Why You Should Consider a Lawsuit for a Faulty DePuy Hip Replacement
A faulty hip replacement can cause years of physical, emotional, and financial damage. The pain it inflicts is often excruciating.
It may prevent you from living your normal life or even from going to work, which also means lost income.
The inability to perform everyday activities and spend time with your family takes also takes a very real toll, for which DePuy should be held accountable.
Lawsuits
If you or a loved one has suffered due to a faulty hip replacement from DePuy, you have options. Should you choose to file a suit against DePuy, you will not be the first; many have already successfully held the company liable for damages related to extra healthcare costs, as well as for pain and suffering.
If you have a case, an experienced medical malpractice lawyer from Gruber Law Offices, LLC can help you show that DePuy produced a dangerously faulty device, and was negligent in its failure to accurately convey the risks involved. If you have a case, an experienced Milwaukee medical malpractice lawyer from Gruber Law Offices, LLC can help you show that DePuy produced a dangerously faulty device, and was negligent in its failure to accurately convey the risks involved.
You deserve to be heard, and you deserve to be compensated for the suffering you or loved ones have undergone following a DePuy hip replacement.
Sources
- DePuy Synthes: ASR Hip Replacement Recall Guide
- American Academy of Orthopaedic Surgeons: Questions and Answers About Metal-on-Metal Implants











